7. Device & Drug Updates
FDA clears reader for next-gen Abbott FreeStyle Libre 3
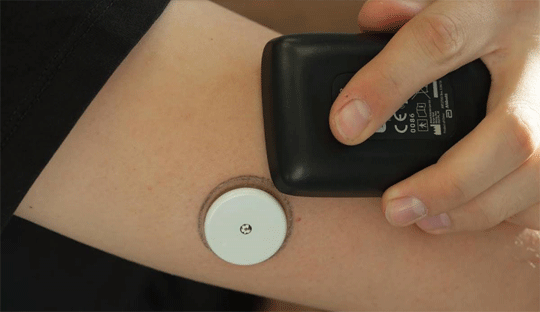
Abbott's FreeStyle Libre 3, has received FDA approval for its reader device, making it a breakthrough in diabetes management. This state-of-the-art continuous glucose monitoring (CGM) system is designed to be discreet and convenient, allowing users to wear it unnoticeably on the back of their upper arm.
The FreeStyle Libre 3 reader is a small, handheld device that provides real-time glucose readings directly from the tiny sensor worn on the upper arm. The readings are displayed on a large, bright, and easy-to-see screen, making it simple for users to manage their diabetes on the go. The compact size of the reader makes it convenient to carry and use, giving users the flexibility to monitor their glucose levels discreetly in any setting. The reader has recorded a mean absolute relative difference (MARD) of 7.9%, which is a remarkable achievement in CGM technology. This high level of accuracy ensures wider acceptance of the device among the users to use it for obtaining precise and reliable glucose readings, enabling them to make informed decisions on their diabetes management.
In addition, users of the FreeStyle Libre 3 system can usually continue to use the existing smartphone apps, providing them with even more options for monitoring their glucose levels. This seamless integration with smartphone apps allows users to track their glucose readings conveniently and easily, using a device that they are already familiar with.
Insulin degludec noninferior to insulin detemir for pregnant women with type 1 diabetes

Insulin degludec, a second-generation basal insulin with an improved pharmacokinetic-pharmacodynamic profile, has been the subject of a non-inferiority trial (EXPECT) conducted by the Department of Clinical Medicine, University of Copenhagen. The trial aimed to assess the safety of insulin degludec in pregnant women with type 1 diabetes, and the results are promising.
The trial, which took place at 56 hospitals and medical centers in 14 countries, enrolled pregnant women who were at least 18 years old and between 8 and 13 weeks gestational age, or who were planning to become pregnant. The participants were randomly assigned in a 1:1 ratio to receive either insulin detemir (100 U/mL) once or twice daily, or insulin degludec (100 U/mL) once or twice daily, both in combination with mealtime insulin aspart (100 U/mL). The trial drug was administered throughout pregnancy and up to 28 days after delivery.
The primary endpoint of the trial was the change in HbA1c measurementsand the results showed that the use of insulin degludec was non-inferior to that of insulin detemir in pregnant women with type 1 diabetes which conveys that insulin degludec is as effective as insulin detemir in maintaining glucose control during pregnancy.
Furthermore, the trial did not highlight any new safety concerns associated with insulin degludec. The researchers noted that these findings are reassuring, as insulin detemir has been well studied and is considered effective with a good safety profile in pregnant populations. This indicates that insulin degludec can be a safe and viable option for pregnant women with type 1 diabetes, providing them with an alternative treatment option for managing their glucose levels during pregnancy.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




