7. Drug Updates
FDA approves Kerendia to reduce risk of serious kidney and heart complications in people with T2D
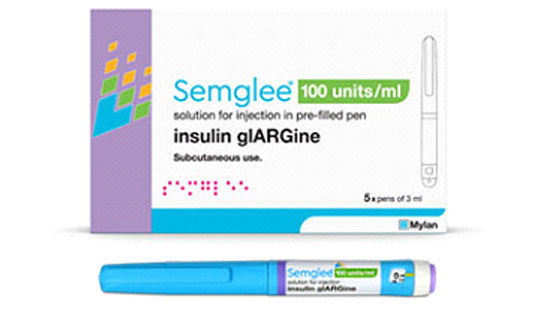
FDA has approved the first interchangeable biosimilar insulin product, indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and adults with type 2 diabetes. Semglee (insulin glargine-yfgn) is both biosimilar to, and interchangeable with its reference product Lantus (insulin glargine), a long-acting insulin analogue. Semglee is the first interchangeable biosimilar product approved in the U.S. for the treatment of diabetes. Semglee offered in 10 mL vials and 3 mL prefilled pens, is administered subcutaneously once daily. The dosing of Semglee should be individualized based on the patient’s needs. Approval of these insulin products can provide patients with additional safe, high-quality, and potentially cost-effective options for treating diabetes.
Sotagliflozin reduces heart attack, heart failure and stroke in diabetics

FDA has approved exenatide extended-release (BYDUREON BCise) for improving glycemic control in pediatric patients aged 10-17 years with type 2 diabetes as an adjunct to exercise and diet. Exenatide extended-release becomes the first once-weekly GLP-1 receptor agonist treatment to receive approval in pediatric populations. The approval is based on the results of the 24-week, randomized, double-blind, placebo-controlled Phase III trial with a 28-week open-label extension, BCB114.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




