
7. Drug & Device Updates
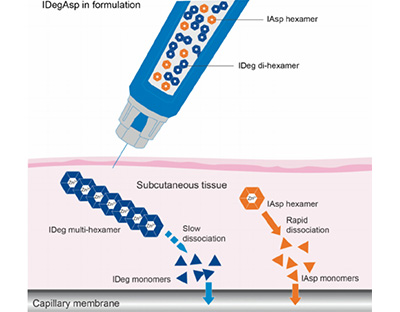
An Evaluation Of The Prescription Pattern Of IDegAsp And Its Clinical Outcomes In Indian Clinical Practice
The study was presented from Jothydev’s Diabetes Research Center, Trivandrum at the International Diabetes Federation Congress 2019, organized by IDF at Busan. The Research team headed by Dr.Jothydev Kesavadev included Dr.Banshi Saboo, Dr.Shashank R Joshi, Dr.Arun Shankar, Dr. Ashwin David Ashok, Lakshmy Ramachandran and Sunitha Jothydev.
IDegAsp, the first soluble co-formulation which contains 70% basal IDeg and 30% mealtime IAsp, has a unique pharmacodynamic profile. Postprandial and fasting hyperglycemia can be effectively controlled, without increasingthe hypoglycaemia risk. IDeg component provides a stable basal insulin action over a 24-h period whereas the IAsp component bestows prandial control, which is unaffected by the basal component.
This study is a retrospective evaluation of the prescription pattern and the clinical outcomes of IDegAsp among T2DM subjects in a real-world setting of Indian clinical practice. Clinical characteristics and demographics of T2DM subjects prescribed with IDegAsp and on regular follow-up were captured from our EMRs. There were 291 participants with mean age of 53.59 ± 11.80 years, and T2DM duration of 11.05 ± 6.28 years. About 74.14% of the study participants were males. IDegAsp treatment duration as on 1st April 2018 was 10.51 ± 8.53 months. Previous treatment regimen of the patients were OHA only, n= 87; OHA + insulin, n=176; OHA + insulin + GLP1RA, n=7 [3 patients discontinued GLP1RA upon IDegAsp initiation], and treatment naive [started on IDegAsp + OHAs, n=17; started on IDegAsp+OHAs+GLP1RA, n=4].
IDegAsp treatment resulted in significant improvement in the clinical profiles. Overall reduction from baseline: FBS- 34.93 mg/dl, p<0.0001; PPBS- 65.09, p<0.0001; HbA1c- 1.470, p<0.0001. Changes in body weight, BMI and TDD of insulin were non-significant. Negligible number of hypoglycaemic episodes reported (0.007 events/person, non severe, no nocturnal hypos). Initially, 32.88% and 67.12% of the subjects were on IDegAsp once daily (q.d) and twice daily (b.i.d) respectively. Later, 22.92 % of the subjects in the q.d regimen had to be intensified to b.i.d and 3.57% of the subjects in the b.i.d were shifted to q.d.
In this real-world study of Indian clinical practice, prescription data indicated that the IDegAsp co-formulation is effective across a range of clinical profiles including treatment naive subjects. It significantly improved clinical outcomes, with negligible hypoglycaemia and no weight gain. To a great extent, IDegAsp could thus help eliminate the concerns regarding insulin intensifications and achieve clinical outcomes in a large proportion of T2DM individuals.
FDA authorizes first interoperable, automated insulin dosing controller
The U.S. FDA authorized marketing of the Tandem Diabetes Care Control-IQ Technology, an interoperable automated glycemic controller device that automatically adjusts insulin delivery by connecting to an alternate controller- enabled insulin pump (ACE pump) and integrated continuous glucose monitor (iCGM). This is the first such controller that can be used with other diabetes devices that are also designed to be integrated into a customizable diabetes management system for automated insulin delivery.
This FDA authorization paves the way for iCGMs and ACE pumps to be used with an interoperable automated glycemic controller as a complete automated insulin dosing (AID) system. AID systems typically consist of a pump, CGM and software to control the system of compatible devices.
The Control-IQ hybrid closed loop algorithm aims is to keep users between 70-180 mg/dl as much as possible using a combination of automated basal insulin delivery and automated correction boluses. These decisions are based on readings from the CGM, which inform the algorithm’s decisions every five minutes. The algorithm includes the following components: Automated Basal Rate Adjustment during the day, Automated Basal Rate Adjustment at night, Automated Bolus Corrections and Exercise Mode.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
