7. Device & Drug Updates
Rezvoglar approved by FDA

FDA approves Lilly’s new basal insulin, Rezvoglar (insulin glargine-aglr), which is a cheaper alternative to Lantus. It is a new long-acting basal insulin for adults and children with type 1 diabetes and adults with type 2 diabetes. Rezvoglar was approved as a biosimilar to Sanofi’s Lantus (insulin glargine) which means that there were no clinically meaningful differences between the glucose lowering effects of both the insulins. According to the package insert, Rezvoglar is available as a 3 mL prefilled KwikPen. As a biosimilar product, Rezvoglar can be used in place of Lantus; however, patients would need a prescription specifically written for Rezvoglar as the products are not considered interchangeable.
Dexcom G7: What’s new?

Dexcom’s highly anticipated G7 continuous glucose monitoring (CGM) system will be an all-in-one device, sensor and transmitter both, and is fully disposable. The 2-hour warm-up time will be reduced to a mere 30 minutes for this tiny sensor. The G7 is factory-calibrated. . G7 will be even easier to apply than the G6, and the applicator will be much smaller than the G6.. G7 is interoperable with hybrid close loop systems and will also integrate with the Apple Watch. G7 is also interoperable with Tandem’s Control IQ and Insulet’s OmniPod system, Companion Medical’s InPen and other commercially-available apps.
MiniMed 780G system
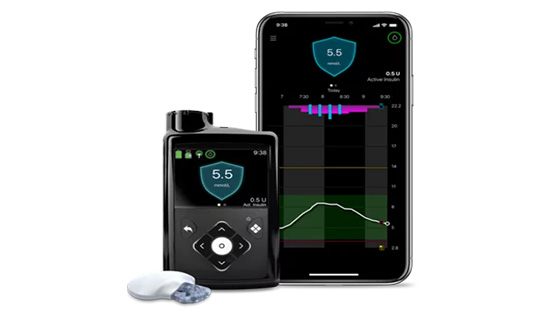
The MiniMed 780G system includes an Advanced Hybrid Closed Loop (AHCL) algorithm which provides for both automated basal and correction bolus insulin delivery. It features an advanced level of automation for diabetes management, known as SmartGuard technology. The MiniMed 780G system automatically adjusts the delivery of basal insulin based on Continuous Glucose Monitoring (CGM) values. The pump gets glucose readings from the CGM (Guardian Sensor 4)and transmitter automatically and then delivers a variable rate of insulin, 24 hours a day based on personal needs. This integrated system (a pump and a CGM) helps to stabilize the glucose levels and reduce both high and low glucose levels. 780 G tracks the glucose levels and provides alarm and notification on the connected smartphone (via CareLink Connect app). It is protected against the effects of being underwater to a depth up to 3.6 meters for up to 24 hours. Although it’s designed to be waterproof, drops and bumps that occur over time can make it more vulnerable to damage from water. The sensor and transmitter are water-resistant at 2.4 meters for up to 30 minutes.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




