7. Device Update
FDA Clears Tandem’s Mobi Insulin Pump
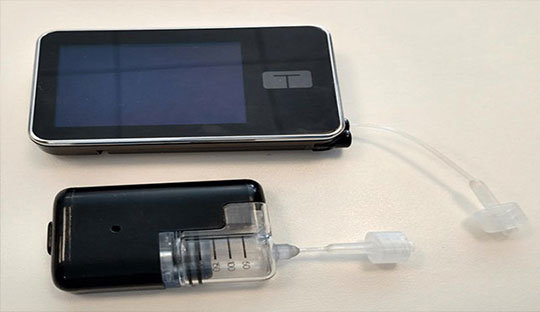
Tandem's latest innovation, the Mobi automated insulin delivery system, has received FDA clearance for use in people aged 6 and older with diabetes. Notably, it is the world's smallest insulin delivery system that is fully controlled by a smartphone. The Mobi is impressively compact, being less than half the size of Tandem's current hybrid closed-loop system, the t:slim X2, and features a 5-inch tubing option.
Unlike the t:slim X2, the Mobi does not include a built-in screen; instead, it is entirely controlled through an iPhone app. Users have the convenience of an on-pump button as an alternative for quickly bolusing insulin if they prefer not to do it from their phone. Despite its small size, the insulin pump can hold a 200-unit insulin cartridge and can easily fit in a coin pocket.
Additionally, users have the flexibility to clip it to clothing or wear it with an armband or adhesive sleeve, offering discreet wearability options.
The Mobi insulin pump boasts water resistance, supports wireless charging, and is capable of receiving remote software updates, making it a cutting-edge and user-friendly device for insulin management. With this new technology, Tandem aims to provide people with diabetes with more convenient and discrete options for insulin delivery and control.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




