3. iLet shows promising results as the closest Artificial Pancreas
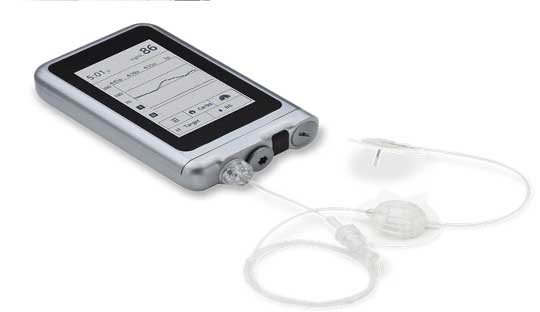
Results from Beta Bionics’s iLet Bionic Pancreas pivotal trial was presented at the American Diabetes Association’s 82nd Scientific Sessions. The iLet Bionic Pancreas system uses a tubed insulin pump and houses the AID algorithm, a Dexcom G6, and a smartphone or other device. It only requires users to enter their body weight to initialize therapy and would then begin to automatically titrate and infuse insulin without requiring the user to count carbohydrates, set insulin-to-carbohydrate ratios, set insulin basal rates, set correction factors, or determine bolus insulin for meals or corrections.
The Insulin-Only Bionic Pancreas Pivotal Trial (IO BPPT) was a pivotal trial assessing the safety and efficacy of the iLet Bionic Pancreas, The pivotal trial included 40 participants included (165 children and teens and 275 adults). At the initial stages of the trial, 88% of the participants were already using a continuous glucose monitor (CGM), and 31% were already on a hybrid closed-loop AID system.
The participants were randomly assigned into one of three different treatment groups:
- Those who continued their standard insulin delivery method (whatever technology they were using at the start) with a Dexcom G6 CGM – that included both children and adults
- Those using the bionic pancreas with Humalog and Novolog insulin – that also included both children and adults
- Those using the bionic pancreas with Fiasp insulin – that had adults only
iLet showed significant improvements in A1C and Time in Range even when compared to people who were already on a hybrid closed-loop AID system such as Control-IQ or MiniMed 670G. In the adult group that was using the bionic pancreas with Fiasp, results were almost identical to the other adult group using the bionic pancreas with Humalog and Novolog. The only major difference was a minor increase in the number of participants achieving a Time in Range greater than 70% (58% of participants in the bionic pancreas with Fiasp group and only 47% in the bionic pancreas with Humalog/Novolog group). There were minimal severe adverse events in all groups – with only a few cases of severe hypoglycemia.
The Bihormonal iLet Bionic Pancreas AID system is also being tested by Beta Bionics.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




