6. Drug Update
New drug Danuglipron from Pfizer shows promise
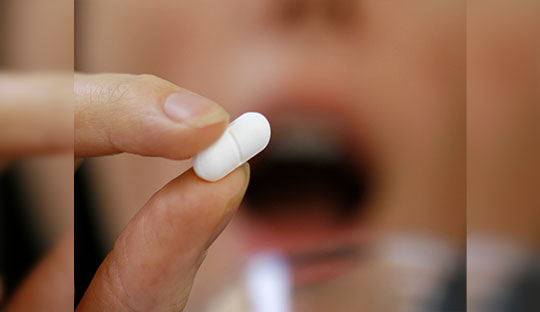
Pfizer is continuing the clinical development of danuglipron, a novel oral GLP-1 medication for type 2 diabetes and obesity. Danuglipron's phase 2 trial has produced encouraging preliminary findings on A1C and weight loss. Over the course of a 16-week period, participants receiving danuglipron noticed dose-dependent decreases in A1C of up to 1.16%, fasting plasma glucose levels of up to 33.24 mg/dL, and body weight of 9 pounds. These favourable outcomes, along with modest adverse effects that are comparable to those of other GLP-1 drugs, imply that danuglipron might be a useful therapeutic choice. Danuglipron might be an extra alternative for persons with type 2 diabetes and obesity if it is authorized.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




