
3. Insulin-and-pramlintide artificial pancreas proven effective
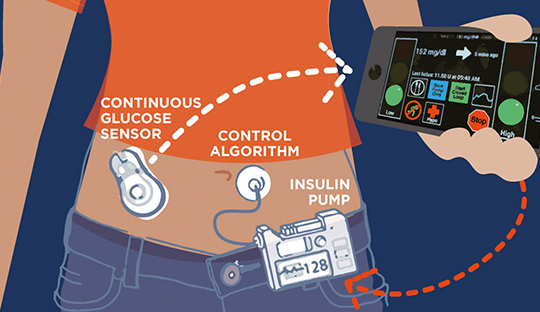
Researches on the effect of insulin therapies for type 1 patients revealed that the existing insulin pumps and artificial pancreas systems still face limitations and hence require add-on features for its effective usage. A recent study by the Mc Grill University researchers observed that even though the rapid insulin-alone artificial pancreas improves glycemia in type 1 diabetes, the daytime control remains suboptimal in subjects. As per this observation, the research team proposed novel dual-hormone artificial pancreas systems for a better glycemic profile in type 1 subjects.
The study was designed as a randomized crossover trial and compared rapid insulin-alone artificial pancreas system with rapid insulin-and-pramlintide and with regular insulin-and-pramlintide artificial pancreas systems in adults with type 1 diabetes. Participants were assigned to the interventions in random order during three 24-h inpatient visits. Each visit was preceded by an outpatient hormonal open-loop run-in period of 10–14 days. The dual-hormone artificial pancreas delivered pramlintide in a basal-bolus manner, using a novel dosing algorithm, with a fixed ratio relative to insulin. The primary outcome was time in the range 3.9–10.0 mmol/L.
The results showed that compared with the rapid insulin-alone artificial pancreas system, the rapid insulin-and-pramlintide system increased the time in range from 74% (SD 18%) to 84% (13%) (P = 0.0014), whereas the regular insulin-and-pramlintide system did not change the time in range (69% [19%]; P = 0.22). The increased time in range with the rapid insulin-and-pramlintide system was due to improved daytime control (daytime time in range increased from 63% [23%] to 78% [16%], P = 0.0004). There were also observed 11 (1 per 2.5 days) hypoglycemic events with the rapid insulin-alone system, compared with 12 (1 per 2.3 days) and 18 (1 per 1.4 days) with the rapid and regular insulin-and-pramlintide systems, respectively. Gastrointestinal symptoms were reported after 0% (0 of 112) of meals with the rapid insulin-alone system, compared with 6% (6 of 108) and 11% (11 of 104) with the rapid and regular insulin-and-pramlintide systems, respectively and none of the symptoms were considered severe.
Thus the study concludes that this novel rapid insulin-and-pramlintide artificial pancreas improves glucose control compared with a rapid insulin-alone artificial pancreas in adult patients with type 1 diabetes.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
