
7. Device Update
FDA approval for the MiniMed 770G system
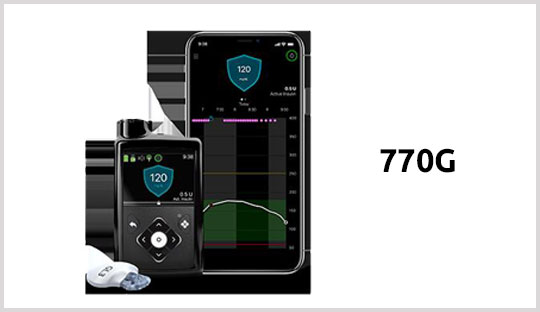
Medtronic's MiniMed 770G hybrid closed loop system got cleared by the FDA for people with type 1 diabetes as young as two years of age. This makes the 770G the first automated insulin delivery (AID) system for children between two and six years old.
The pump in the 770G system is Bluetooth-connected, that allows users to view pump data on their phones and upload data or update their pump wirelessly. With the help of MiniMed smartphone app, users can easily share glucose and insulin data with their care-partners for remote monitoring, as well as with healthcare professionals and diabetes care and education specialists. It will also provide in-app alerts when blood glucose levels are out of range.
The FDA evaluated data from a clinical trial that included 46 children aged 2 to 6 years old with Type 1 diabetes. Study participants wore the device for approximately 3 months to evaluate its performance while at home and at a hotel to stress the system with sustained daily exercise. The study found no serious adverse events and that the device is safe for use. Data from that study was used to help support the expanded indication for patients 2 to 6 years old.
780G Advanced Hybrid Closed Loop shows promising results

MiniMed 780G system, which features a default target of 100mg/dL (with the option of 120mg/dL), programmable insulin action time from two to eight hours, and automatic corrections every five minutes, met all study endpoints and demonstrated high user satisfaction across the studies presented at ADA 2020 Virtual conference.
Results of the 90-day at home U.S. pivotal trial, studying adults and adolescents aged 14-75 years old, show the trial successfully met both safety and glycemic endpoints and demonstrated no occurrences of severe adverse events with an overall Time in Range of 75%, with overall Time Below Range of 1.8% and overnight Time in Range of 82%, with overnight Time Below Range of 1.5%. Results from a study questionnaire also demonstrated high user satisfaction with 96% indicating it was easy to use.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
