
2. Insulin sparing effects of Sitagliptin
![]()
 This study was presented by Jothydev’s Diabetes Research Centre at the 75th ADA Scientific Sessions at Boston this June.
The study team was led by Dr.Jothydev Kesavadev.
This study was presented by Jothydev’s Diabetes Research Centre at the 75th ADA Scientific Sessions at Boston this June.
The study team was led by Dr.Jothydev Kesavadev.
![]() Addition of Sitagliptin to treatment of T2DM patients poorly controlled on insulin +/- metformin has been shown to reduce HbA1c while being generally well-tolerated. We conducted a 24 weeks randomized, open-label, active-controlled, parallel-arm study, with a 6-week dose titration period (to achieve maximum tolerable dose of glimepiride:1- 3mg per day) and 18-week maintenance period to study effect of Sitagliptin(SG), compared to glimepiride(GM), in T2DM patients on treatment with metformin and insulin with HbA1c values ≥7.3 % to ≤8.5%. Glycemic endpoints, hypoglycemia, weight change, reduction in the total daily dose (TDD) of insulin, cardio vascular risk factors, change in c-peptide levels and HOMA-β and HOMA-IR were assessed.
Addition of Sitagliptin to treatment of T2DM patients poorly controlled on insulin +/- metformin has been shown to reduce HbA1c while being generally well-tolerated. We conducted a 24 weeks randomized, open-label, active-controlled, parallel-arm study, with a 6-week dose titration period (to achieve maximum tolerable dose of glimepiride:1- 3mg per day) and 18-week maintenance period to study effect of Sitagliptin(SG), compared to glimepiride(GM), in T2DM patients on treatment with metformin and insulin with HbA1c values ≥7.3 % to ≤8.5%. Glycemic endpoints, hypoglycemia, weight change, reduction in the total daily dose (TDD) of insulin, cardio vascular risk factors, change in c-peptide levels and HOMA-β and HOMA-IR were assessed. 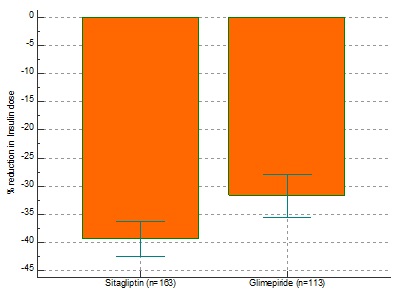 Of the 440 patients (219 on SG, 221 on GM), 418 patients completed the study. Mean age 51.09(6.58), mean duration 14.96(7.33), mean A1c 7.96(0.33).
Of the 440 patients (219 on SG, 221 on GM), 418 patients completed the study. Mean age 51.09(6.58), mean duration 14.96(7.33), mean A1c 7.96(0.33).
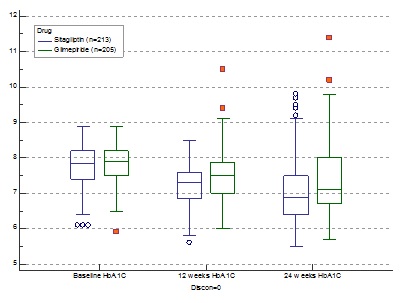
![]() There was a statistically significant reduction in HbA1c from baseline with SG (-0.70/-8.97%) compared to GM(-0.31/-3.98%) after 24 weeks (p<0.0001.).(Fig.1) 25.82% patients on SG achieved an A1C of <6.5% compared to GM (10.73%) therapy (p<0.0001). Our study among 418 patients have shown sitagliptin to be superior to glimepiride in terms of A1c reduction, lower instances of hypoglycaemia and significant reduction in the total daily dose of insulin.
There was a statistically significant reduction in HbA1c from baseline with SG (-0.70/-8.97%) compared to GM(-0.31/-3.98%) after 24 weeks (p<0.0001.).(Fig.1) 25.82% patients on SG achieved an A1C of <6.5% compared to GM (10.73%) therapy (p<0.0001). Our study among 418 patients have shown sitagliptin to be superior to glimepiride in terms of A1c reduction, lower instances of hypoglycaemia and significant reduction in the total daily dose of insulin.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
