7. Drug & Device Update
Imeglimin
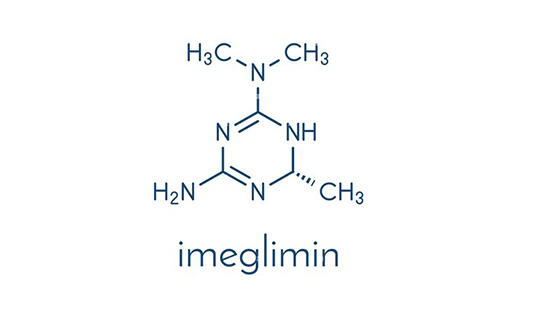
Imeglimin is an orally administered, first-in-class glimin for the treatment of type 2 diabetes. Glimins are a novel class of glucose-lowering agents that target multiple components of diabetes-associated pathology. These oxidative phosphorylation blockers act to inhibit hepatic gluconeogenesis, increase muscle glucose uptake, and restore normal insulin secretion. Currently this drug is approved for use in Japan in 2021 based on extensive preclinical and clinical data, including positive results from the pivotal phase III TIMES programme.
Kerendia (Finerenone)
Kerendia (Finerenone) is a brand new drug approved by FDA on July 2021. It has been shown to slow the progression of chronic kidney disease (CKD) for people with type 2 diabetes. Kerendia belongs to the class of non-steroidal mineralocorticoid receptor antagonists (or MRA). This is the first drug in this class to be approved by the FDA for treating these specific conditions. The drug blocks the overactivation of mineralocorticoid receptor in the kidney cells which in turn reduces fibrosis and inflammation; both of which can cause damage to the kidneys and lead to CKD or kidney failure. It is indicated to reduce the risk of eGFR decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure associated with diabetes related CKD in patients with type 2 diabetes.
Bionic pancreas in type 1 diabetes

Bionic pancreas is an automated insulin-delivery system which is initialized only on the basis of body weight, makes all dose decisions and delivers insulin autonomously, and uses meal announcements without carbohydrate counting. In a 13-week, multicenter, randomized trial, individuals at least 6 years of age with type 1 diabetes are randomized either to receive bionic pancreas treatment with insulin aspart or insulin lispro or to receive standard care (defined as any insulin-delivery method with unblinded, real-time continuous glucose monitoring). The primary outcome was the HbA1c level at 13 weeks and secondary outcome was the percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg/dL. The trial showed a decrease in the HbA1c level from 7.9% to 7.3% in the bionic-pancreas group and did not change (was at 7.7% at both time points) in the standard-care group (mean adjusted difference at 13 weeks, −0.5 percentage points; 95% confidence interval [CI], −0.6 to −0.3; P< 0.001). The percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg/dL and did not differ significantly between the two groups.
The trial highlights the potential benefits of using bionic pancreas for a significant reduction of HbA1c than standard care.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




