
7. Drug Updates |
![]() This original research was presented by Jothydev's Diabetes Research Centers at
the American Association of Clinical Endocrinologists (AACE) 2017 Annual Meeting in Austin, TX.
This original research was presented by Jothydev's Diabetes Research Centers at
the American Association of Clinical Endocrinologists (AACE) 2017 Annual Meeting in Austin, TX.
![]() Objective: Incretin mimetic Liraglutide is a long-acting human GLP-1 analogue with 97% amino acid homology to the human endogenous GLP-1. It is the first once-daily human GLP-1 analogue for type 2 diabetes mellitus (T2DM) individuals and helps to regulate glucose metabolism in a glucose-dependent manner by stimulating insulin secretion from β cells, and by suppressing glucagon release. LEAD programme confirmed the efficacy and safety of LIRA and as compared to many other therapies, it does not increase the risk of hypoglycaemia. We performed a retrospective analysis to assess the treatment outcomes of Victoza among our patients.
Methods: The electronic medical records of T2DM patients enrolled in our diabetes clinic were scrutinised to de-identify the patients who had been treated with LIRA. The inclusion criteria were as follows- new onset T2DM patients (diabetes duration < 1 year) who were initiated on LIRA either alone or along with insulin. Exclusion criteria included patients with history of smoking and alcoholism. A retrospective analysis of their follow-up data was conducted to evaluate the treatment outcomes of LIRA. Statistical analysis (Paired t test) was done using GraphPad Prism version 6.01 for Windows.
Objective: Incretin mimetic Liraglutide is a long-acting human GLP-1 analogue with 97% amino acid homology to the human endogenous GLP-1. It is the first once-daily human GLP-1 analogue for type 2 diabetes mellitus (T2DM) individuals and helps to regulate glucose metabolism in a glucose-dependent manner by stimulating insulin secretion from β cells, and by suppressing glucagon release. LEAD programme confirmed the efficacy and safety of LIRA and as compared to many other therapies, it does not increase the risk of hypoglycaemia. We performed a retrospective analysis to assess the treatment outcomes of Victoza among our patients.
Methods: The electronic medical records of T2DM patients enrolled in our diabetes clinic were scrutinised to de-identify the patients who had been treated with LIRA. The inclusion criteria were as follows- new onset T2DM patients (diabetes duration < 1 year) who were initiated on LIRA either alone or along with insulin. Exclusion criteria included patients with history of smoking and alcoholism. A retrospective analysis of their follow-up data was conducted to evaluate the treatment outcomes of LIRA. Statistical analysis (Paired t test) was done using GraphPad Prism version 6.01 for Windows.
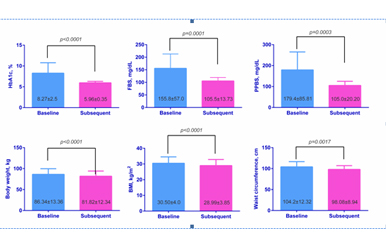
![]() Results: 71 patients were identified with mean age- 40.90±11.25 years, males- 76.06%, mean HbA1c= 8.27±2.5%, mean (Fasting Blood Sugar) FBS= 155.8±57.0mg/dL, mean Post Prandial Blood Sugar (PPBS)= 179.4±85.81 mg/dL, mean Body Mass Index (BMI) = 30.50±4.0kg/m2, mean Body Weight (BW)= 86.34±13.36kg and mean Waist Circumference (WC)= 104.2±12.32cm. LIRA treatment brought out positive results in terms of improvements in various clinical parameters viz. HbA1c was lowered to almost near normal levels of 5.96±0.35% (p<0.0001), FBS to 105.5±13.73mg/dL (p<0.0001), PPBS to 105.0±20.20mg/dL (p=0.0003), BMI to 28.99±3.85kg/m2 (p<0.0001), BW to 81.82±12.34kg (p<0.0001) and WC to 98.08±8.94cm (p=0.0017).
Discussion: LIRA brought about better treatment outcomes in new onset T2DM patients as revealed by improvements in various clinical parameters which in turn can contribute significantly towards reducing the future risk of diabetes-associated complications.
Results: 71 patients were identified with mean age- 40.90±11.25 years, males- 76.06%, mean HbA1c= 8.27±2.5%, mean (Fasting Blood Sugar) FBS= 155.8±57.0mg/dL, mean Post Prandial Blood Sugar (PPBS)= 179.4±85.81 mg/dL, mean Body Mass Index (BMI) = 30.50±4.0kg/m2, mean Body Weight (BW)= 86.34±13.36kg and mean Waist Circumference (WC)= 104.2±12.32cm. LIRA treatment brought out positive results in terms of improvements in various clinical parameters viz. HbA1c was lowered to almost near normal levels of 5.96±0.35% (p<0.0001), FBS to 105.5±13.73mg/dL (p<0.0001), PPBS to 105.0±20.20mg/dL (p=0.0003), BMI to 28.99±3.85kg/m2 (p<0.0001), BW to 81.82±12.34kg (p<0.0001) and WC to 98.08±8.94cm (p=0.0017).
Discussion: LIRA brought about better treatment outcomes in new onset T2DM patients as revealed by improvements in various clinical parameters which in turn can contribute significantly towards reducing the future risk of diabetes-associated complications.
![]() Conclusion: LIRA was found to be an effective and well-tolerated once-daily human GLP-1 analogue and helped to improve over-all glycaemic control (HbA1c, FBS, PPBS) along with significant reductions in BW, BMI and WC.
Conclusion: LIRA was found to be an effective and well-tolerated once-daily human GLP-1 analogue and helped to improve over-all glycaemic control (HbA1c, FBS, PPBS) along with significant reductions in BW, BMI and WC.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
