
7. Device and Drug Updates
Maestro Rechargeable System for Obesity
![]()
 The U.S. Food and Drug Administration (FDA) approved the use of an internal electronic device to combat adult obesity. The Maestro Rechargeable System targets the nerve pathway between the brain and the stomach to control the feelings of hunger and fullness.
The U.S. Food and Drug Administration (FDA) approved the use of an internal electronic device to combat adult obesity. The Maestro Rechargeable System targets the nerve pathway between the brain and the stomach to control the feelings of hunger and fullness.
![]() The rechargeable electric pulse generator, wires and electrodes are implanted in the abdomen and intermittent electrical pulses are send to the trunks of the abdominal nerve that signals the brain. The device is recharged and adjusted by external controllers.
The device is only approved to treat patients 18 and older who have not been able to lose weight by dieting, have a body mass index of 35 to 45 and who have an obesity-related condition like
The rechargeable electric pulse generator, wires and electrodes are implanted in the abdomen and intermittent electrical pulses are send to the trunks of the abdominal nerve that signals the brain. The device is recharged and adjusted by external controllers.
The device is only approved to treat patients 18 and older who have not been able to lose weight by dieting, have a body mass index of 35 to 45 and who have an obesity-related condition like
Type 2 diabetes.
Temporary tattoo measures blood glucose levels
![]()
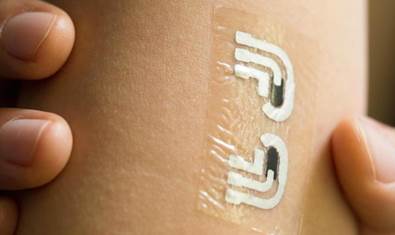 Scientists at the University of California, San Diego, has designed a thin and flexible patch resembling a non permanent tattoo that can consistently check glucose in the blood without puncturing or irritating the pores and skin. The sensor is a very clear patch affixed with two smaller electrodes and an enzyme that reacts with glucose.
Scientists at the University of California, San Diego, has designed a thin and flexible patch resembling a non permanent tattoo that can consistently check glucose in the blood without puncturing or irritating the pores and skin. The sensor is a very clear patch affixed with two smaller electrodes and an enzyme that reacts with glucose.
![]() The researchers ran a mild electrical existing by way of the electrodes to generate glucose to the area of the pores and skin wherever it reacted with the enzyme on the patch. Measuring the response authorized the researchers to precisely take blood glucose measurements in 7 balanced volunteers. The results were release in journal Analytical Chemistry.
The researchers ran a mild electrical existing by way of the electrodes to generate glucose to the area of the pores and skin wherever it reacted with the enzyme on the patch. Measuring the response authorized the researchers to precisely take blood glucose measurements in 7 balanced volunteers. The results were release in journal Analytical Chemistry.
Apple Watch displays live glucose readings from CGM
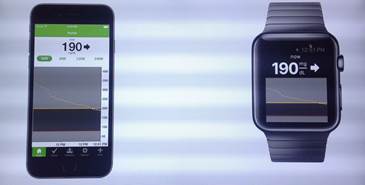
![]() Dexcom revealed an updated design of the Gen 5 CGM user interface integrated directly with an Apple iPhone app and Apple Watch which displays the current blood sugar reading, a graph of recent blood sugars and a trend arrow indicating the glucose trend.
Dexcom revealed an updated design of the Gen 5 CGM user interface integrated directly with an Apple iPhone app and Apple Watch which displays the current blood sugar reading, a graph of recent blood sugars and a trend arrow indicating the glucose trend.
![]() Dexcom announced that its 5th generation system will transfer data from the sensor to the phone which represents a significant upgrade over current G4 solutions which uses the Dexcom Share. Dexcom’s 5th gen release would eliminate the need for a Share-like receiver and could potentially even eliminate the receiver in 4th generation CGM.
Dexcom announced that its 5th generation system will transfer data from the sensor to the phone which represents a significant upgrade over current G4 solutions which uses the Dexcom Share. Dexcom’s 5th gen release would eliminate the need for a Share-like receiver and could potentially even eliminate the receiver in 4th generation CGM.
OneTouch Verio® Blood Glucose Monitoring System
![]()
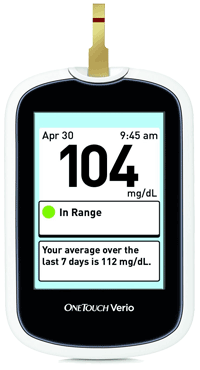 LifeScan, Inc. has introduced the new OneTouch Verio Blood Glucose Monitoring System that features a color-coded range indicator that shows whether a test result is within, below or above the range limits set in the meter without the need to scroll or push buttons. It also looks for signs of progress and provides positive reinforcement with Progress Notes, which inform patients about the progress they are making in managing their diabetes and how often their blood glucose results are in range.
LifeScan, Inc. has introduced the new OneTouch Verio Blood Glucose Monitoring System that features a color-coded range indicator that shows whether a test result is within, below or above the range limits set in the meter without the need to scroll or push buttons. It also looks for signs of progress and provides positive reinforcement with Progress Notes, which inform patients about the progress they are making in managing their diabetes and how often their blood glucose results are in range.
Xultophy launched in Switzerland

![]() Xultophy (IDegLira) which combines ultra-long-acting insulin Tresiba (insulin degludec) with its GLP-1 analogue Victoza (liraglutide) in a once-daily injection has been launched in Switzerland. The new product has been tipped as a potential blockbuster for Novo Nordisk as it is the first combination of an insulin and GLP-1 receptor agonist to reach the market. Combining the two drug classes together improves blood glucose control, while liraglutide's profile helps counteract the side effects typically seen with insulin, such as weight gain and hypoglycaemia.
Xultophy (IDegLira) which combines ultra-long-acting insulin Tresiba (insulin degludec) with its GLP-1 analogue Victoza (liraglutide) in a once-daily injection has been launched in Switzerland. The new product has been tipped as a potential blockbuster for Novo Nordisk as it is the first combination of an insulin and GLP-1 receptor agonist to reach the market. Combining the two drug classes together improves blood glucose control, while liraglutide's profile helps counteract the side effects typically seen with insulin, such as weight gain and hypoglycaemia.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
