
7. Device Updates
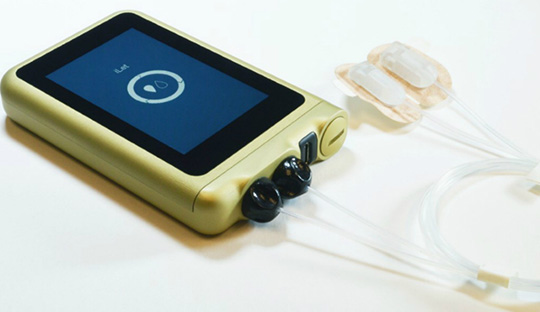
Breakthrough device designation for iLet
The FDA’s breakthrough designation is intended to accelerate regulatory review of a therapy that has potential to address unmet medical needs. According to a press release from Beta Bionics, the FDA has granted breakthrough device designation to an investigational bionic pancreas system, iLet developed by Edward R Damiano, the President and CEO of Beta Bionics.
The iLet is a pocket-sized, wearable medical device designed to autonomously control glucose levels with on-body wear similar to an insulin pump. Unlike insulin pump therapy, the bionic pancreas is designed for users to enter only their body weight for the device to initialize therapy, with no need to count carbohydrates, set insulin delivery rates or deliver bolus insulin for meals or corrections.
Damiano, presented his research findings, said that the mean reduction in glucose level was 162 mg/dL with the bionic pancreas compared with 141 mg/dL with the insulin pump over 11 days (P < .0001). The bionic pancreas was also associated with greater reductions in time less than 60 mg/dL by continuous glucose monitoring (1.9% vs. 0.6%; P < .0001).
The bionic pancreas is designed to function as three medical devices in one. It can be configured as an insulin-only bionic pancreas, a glucagon-only bionic pancreas or a bihormonal bionic pancreas using insulin and glucagon.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
