
7. Drug Updates
First-ever Artificial Pancreas Software Algorithm GlucoSitter software receives CE approval
![]()
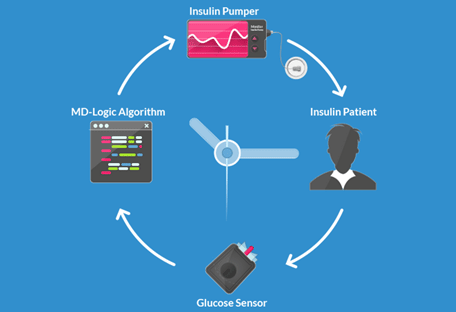 GlucoSitter software received a CE Mark in Europe, making it the first-ever artificial pancreas algorithm to receive regulatory approval in the world. A newly established company, DreaMed Diabetes, is tasked with the commercialization of this algorithm into a full closed-loop system (software algorithm + pump + CGM).
The GlucoSitter software is based on the MD-Logic closed-loop algorithm and is the product of the Diabetes wiREless Artificial Pancreas ConsortiuM (DREAM). Use of the MD-Logic algorithm has significantly decreased patients’ time spent in hypoglycemia and increased their time spent in-range in overnight and 24-hours/day.
GlucoSitter software received a CE Mark in Europe, making it the first-ever artificial pancreas algorithm to receive regulatory approval in the world. A newly established company, DreaMed Diabetes, is tasked with the commercialization of this algorithm into a full closed-loop system (software algorithm + pump + CGM).
The GlucoSitter software is based on the MD-Logic closed-loop algorithm and is the product of the Diabetes wiREless Artificial Pancreas ConsortiuM (DREAM). Use of the MD-Logic algorithm has significantly decreased patients’ time spent in hypoglycemia and increased their time spent in-range in overnight and 24-hours/day.
![]() DreaMed Diabetes has announced that it had signed an exclusive worldwide development and license agreement with Medtronic, the world's premier medical technology and services company, for the development and marketing of products incorporating DreaMed’s MD-Logic Artificial Pancreas algorithm in Medtronic’s insulin pumps.
DreaMed Diabetes has announced that it had signed an exclusive worldwide development and license agreement with Medtronic, the world's premier medical technology and services company, for the development and marketing of products incorporating DreaMed’s MD-Logic Artificial Pancreas algorithm in Medtronic’s insulin pumps.
Saxagliptin May Slow Beta-cell Decline in Type 2
![]() Saxagliptin (Onglyza) is a commonly prescribed dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. An international team of researchers examined the effects of saxagliptin on glycemic stability and β-cell function in the SAVOR-TIMI 53 trial.
Saxagliptin (Onglyza) is a commonly prescribed dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. An international team of researchers examined the effects of saxagliptin on glycemic stability and β-cell function in the SAVOR-TIMI 53 trial.
![]()
 In this multinational double blind trial, 16,492 patients with type 2 diabetes were randomized to either saxagliptin or placebo, which were added to the patients' current antidiabetic medications. The two randomized groups were then followed for a median of 2.1 years.
In this multinational double blind trial, 16,492 patients with type 2 diabetes were randomized to either saxagliptin or placebo, which were added to the patients' current antidiabetic medications. The two randomized groups were then followed for a median of 2.1 years.
![]() When compared with placebo, the participants treated with saxagliptin were shown to have a reduction in the development of glycemic instability. In participants treated with saxagliptin when compared with placebo, the occurrence of an HbA1c increase of ≥0.5% resulted in a reduction by 35.2%; the initiation of insulin was decreased by 31.7% and the increases in doses of any oral anti-diabetic drug or insulin were reduced by 19.5 and 23.5%, respectively. At the 2 year mark, HOMA-2β values decreased by 4.9% in subjects who were treated with placebo, compared with an increase of 1.1% in those treated with saxagliptin (p<0.0001).
When compared with placebo, the participants treated with saxagliptin were shown to have a reduction in the development of glycemic instability. In participants treated with saxagliptin when compared with placebo, the occurrence of an HbA1c increase of ≥0.5% resulted in a reduction by 35.2%; the initiation of insulin was decreased by 31.7% and the increases in doses of any oral anti-diabetic drug or insulin were reduced by 19.5 and 23.5%, respectively. At the 2 year mark, HOMA-2β values decreased by 4.9% in subjects who were treated with placebo, compared with an increase of 1.1% in those treated with saxagliptin (p<0.0001).
![]() The researchers concluded that saxagliptin did show improvement in glycemia and it also prevented the reduction in HOMA-2β values. Therefore, saxagliptin may help reduce the usual decline in β-cell function in type 2 diabetes patients, thereby slowing the progression of diabetes.
The researchers concluded that saxagliptin did show improvement in glycemia and it also prevented the reduction in HOMA-2β values. Therefore, saxagliptin may help reduce the usual decline in β-cell function in type 2 diabetes patients, thereby slowing the progression of diabetes.
New safety labeling for Onglyza recommended
The Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) of FDA voted in favor of including new safety information for Onglyza (saxagliptin) following EMDAC review of the results of the SAVOR trial, which found a 27% increase in the rate of hospitalization for heart failure, as well as an increase in all-cause mortality, in patients who had taken the drug.
The majority of the panel opted not to vote in favor of more serious actions, including label changes with restricted distribution of the drug or withdrawing saxagliptin from the market. The panel also voted that the SAVOR trial demonstrated saxagliptin has an acceptable CV risk profile.
Some panelists said the findings were difficult to interpret and called for more long-term studies, noting that the SAVOR trial included only participants who were already at a high risk for heart disease and may not apply to all patients with type 2 diabetes.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
